Research
As target-specific biopharmaceuticals gain attention as a new medical modality, the drug delivery system (DDS) field is undergoing a paradigm shift from conventional small-molecule compounds to biopharmaceuticals. However, when artificially synthesized polymeric materials are used as DDS carriers, they may be detected by various cellular and extracellular sensors and recognized as foreign substances. In contrast, cells naturally communicate through biopolymers such as proteins, nucleic acids, and carbohydrates. Understanding and responding to these biological communications is essential for designing effective and safe medical materials. Our goal is to develop novel biofunctional materials that leverage diverse recognition mechanisms both inside and outside the cells.
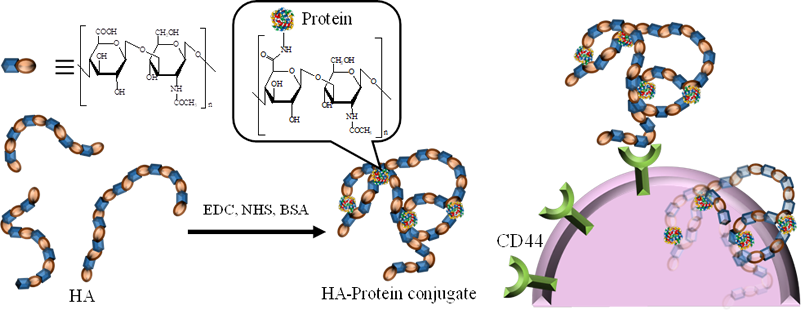
The antigens used in cancer vaccines are not highly antigenic, partly because they are derived from self-proteins. Enhancing the antigenicity of cancer cells is expected to improve the efficacy of cancer vaccines. Since cancer cells overexpress CD44, a receptor for hyaluronic acid (HA), conjugating HA with an antigen (a protein or peptide) enables the targeted delivery of the antigen to cancer cells via HA. Our goal is to modify antigenicity by replacing the “self” antigens presented by cancer cells with “non-self” antigens through the delivery of foreign proteins or peptides that function as antigens.
● Representative papers
- S. Ogata, R. Tsuji, A. Moritaka, S. Ito, *S. Mochizuki. Modification of antigenicity of cancer cells by conjugate consisting of hyaluronic acid and foreign antigen. Biomater. Sci., 11(17), 5809-5818 (2023)
- R. Tsuji, S. Ogata, *S. Mochizuki. Interaction between CD44 and highly condensed hyaluronic acid through crosslinking with proteins. Bioorg. Chem., 121, 105666 (2022)
S. Ogata, R. Tsuji, A. Moritaka, S. Ito, S. Mochizuki. Biomater. Sci., 2023
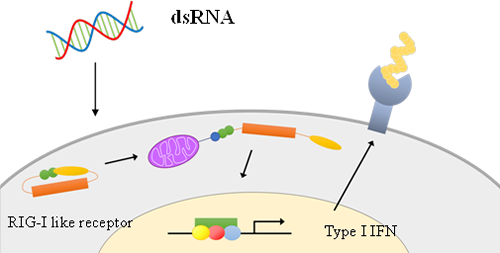
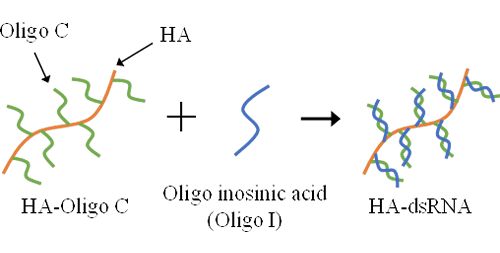
Cancer cells are recognized and attacked by cytotoxic T cells (CTLs), which are activated by vaccine administration. However, cancer cells can evolve to evade CTL-mediated immune responses. One such mechanism involves the downregulation of major histocompatibility complex (MHC) class I molecules. CTLs rely on cancer antigens presented on MHC class I molecules as landmarks to identify and attack cancer cells. When the expression of MHC class I molecules is suppressed, CTLs lose their ability to recognize and eliminate cancer cells. This phenomenon is particularly prevalent in skin cancer (melanoma), which often lacks MHC class I expression. Cells have various nucleic acid sensors, and can be activated by stimulating these sensors with appropriate nucleic acids, resulting in the induction of MHC class I expression. We are working to enhance the sensitivity of cancer cells to the immune system by creating conjugates consisting of hyaluronic acid and nucleic acids, specifically, double-stranded RNA.
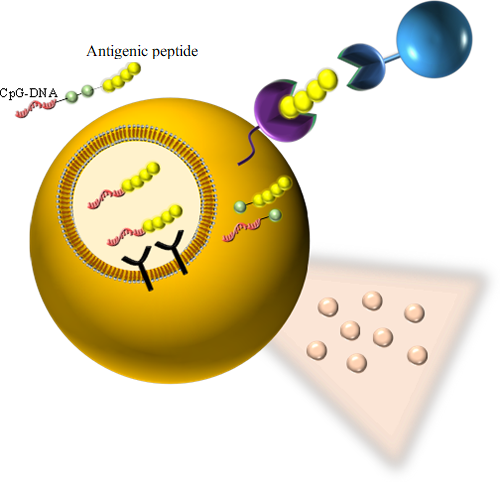
We aim to induce antigen-specific immunity using a conjugate that chemically links an antigenic peptide to an immunostimulatory nucleic acid (CpG-DNA). The conjugate is designed to (1) be recognized by Toll-like receptor 9 (TLR9) in endosomes, (2) release the peptide into the cytoplasm, and (3) efficiently bind to MHC class I molecules in the endoplasmic reticulum (ER) for antigen presentation following cellular uptake.
● Representative papers
- H. Irie, K. Morita, M. Matsuda, M. Koizumi, *S. Mochizuki. Tyrosinase-related protein2 peptide with replacement of N-terminus residue by cysteine binds to H-2Kb and induces antigen-specific cytotoxic T lymphocytes after conjugation with CpG-DNA. Bioconj. Chem., 34(2), 433-442 (2023)
- H. Irie, K. Morita, M. Koizumi, *S. Mochizuki. Immune responses and antitumor effect through delivering to antigen presenting cells by optimized conjugates consisting of CpG-DNA and antigenic peptide. Bioconj. Chem., 31(11), 2585-2595 (2020)